Abstract
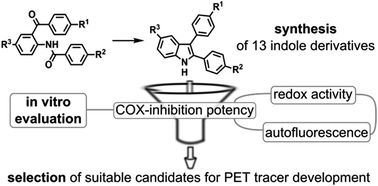
A series of 2,3-diaryl-substituted indoles containing a fluorine or methoxy group was synthesized via Fischer indole synthesis, McMurry cyclization, or Bischler–Möhlau reaction to identify potential leads for positron emission tomography (PET) radiotracer development as well as for optical imaging. All 2,3-diaryl-substituted indoles possess autofluorescent properties with an emission maximum in a range of 443–492 nm, which is acceptable for biological studies in vitro and, in part, in vivo. The molecular structure of compounds 3a and 3j was confirmed by X-ray crystal structure analysis. COX inhibitory activity was evaluated by a fluorescence-based and enzyme immunoassay-based assay. Redox activity of all target compounds was also determined. All synthesized 2,3-diaryl-substituted indoles are inhibitors of COX-2 enzyme in the low micromolar range. Compounds 3e, 3f, 3g and 3m displayed a 30–40% inhibition of COX-2 at 0.1 μM concentration while compounds 3f and 3g also exhibited COX-1 inhibitory activity. Various compounds like 3g showed substantial antioxidative potential (RDIENE = 2.85, RHAVA = 1.98), an effect that was most measurable with methoxy-substituted compounds. With respect to PET radiotracer synthesis, OMe-containing compound 3j was selected as a promising candidate for carbon-11 labeling, and F-containing compound 3m as a lead for the development of a fluorine-18 labeled derivative.

 Please wait while we load your content...
Please wait while we load your content...