Selective oxidation of glycerol to formic acid in highly concentrated aqueous solutions with molecular oxygen using V-substituted phosphomolybdic acids
Abstract
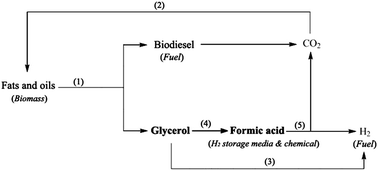
Formic acid is an important commodity chemical as well as a promising medium for hydrogen storage and hydrogen production. In this paper, we report that formic acid can be produced through selective oxidation of glycerol, a low-cost by-product of biodiesel, by using vanadium-substituted phosphomolybdic acids as catalysts and molecular oxygen as the oxidant. Significantly, this catalytic system allows for high-concentration conversions and thus leads to exceptional efficiency. Specifically, 3.64 g of formic acid was produced from 10 g of glycerol/water (50/50 in weight) solution.

 Please wait while we load your content...
Please wait while we load your content...