NiO nanostructures: synthesis, characterization and photocatalyst application in dye wastewater treatment
Abstract
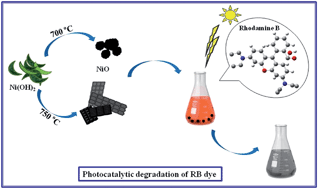
Nickel oxide (NiO) nanostructures have been prepared via a thermal decomposition method. Nanostructures were prepared by calcining β-Ni(OH)2 at various temperatures. H2(pnAA2), 1,3-propylenediamine, nickel nitrate, NaOH and acetyl acetonate were applied as starting reagents to fabricate the NiO nanostructures. The band gap was 2.83 eV, confirming the semi-conductive nature of the prepared NiO nanostructures and indicating its potential as a photocatalyst in effluent treatment. UV irradiation times, quantity of catalyst, pH and dye concentration were investigated by degrading Rhodamine B (RB, C28H31N2O3Cl) dye. These crucial factors indicated that the NiO nanostructures are an effective photocatalyst. Kinetic investigations of photodegradation revealed that the reactions followed the improved Langmuir–Hinshelwood model. The as-produced nanostructures were characterized using XRD, FESEM, FT-IR, UV-vis, VSM and BET.
- This article is part of the themed collections: Nanomaterial applications and Water treatment

 Please wait while we load your content...
Please wait while we load your content...