Oxovanadium(iv) catecholates of terpyridine bases for cellular imaging and photocytotoxicity in red light†
Abstract
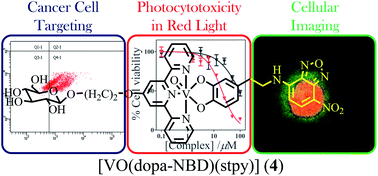
Oxovanadium(IV) catecholates of terpyridyl bases, viz. [VO(cat)(L)] (L = phtpy, 1; stpy, 2) and [VO(dopa-NBD)(L)] (L = phtpy, 3; stpy, 4), where cat is benzene-1,2-diolate, dopa-NBD is 4-(2-(4-nitrobenzo[c][1,2,5]oxadiazol-7-ylamino)ethyl)benzene-1,2-diolate, phtpy is (4′-phenyl)-2,2′:6′,2′′-terpyridine and stpy is (2,2′:6′,2′′-terpyridin-4′-oxy)ethyl-β-D-glucopyranoside, were prepared and characterized, and their DNA binding, DNA photo-cleavage activity, photocytotoxicity in red light (600–720 nm), cellular uptake and intracellular localization behaviour were studied. The complexes showed an intense ligand-to-metal charge transfer (LMCT) band at ∼500 nm. The sugar appended complexes 2 and 4 showed significant uptake into the cancer cells. The dopa-NBD complexes 3 and 4 showing green emission were used for cellular imaging. The complexes showed diffused cellular localization mainly in the cytosol and to a lesser extent into the nucleus as evidenced from the confocal microscopy study. Complexes 1–4 showed significant photocytotoxicity in the PDT spectral window giving low IC50 values, while remaining relatively non-toxic in dark.

 Please wait while we load your content...
Please wait while we load your content...