Long-alkyl-chain-derivatized imidazolium salts and ionic liquid crystals with tailor-made properties†
Abstract
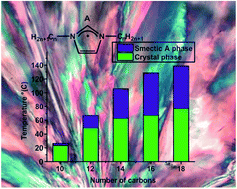
A series of new 1,3-dialkylimidazolium salts with the general formula [CnCnIM][A] (for n = 12; A = PF6−, OTf−, NTf2−; for n = 10, 14, 16, and 18; A = BF4−, ClO4−) has been synthesized. The [C12C12IM][A] salts are ionic liquids at room temperature, whereas all [CnCnIM][BF4] and [CnCnIM][ClO4] (n = 10, 14, 16, 18) salts demonstrate a liquid crystalline phase at elevated temperatures that have a large mesophase window, which varies from 10 to 80 °C with increasing alkyl chain length. In particular, [C10C10IM][BF4] shows liquid crystalline behavior at room temperature and therefore is potentially suitable for application as a pre-organized reaction medium in synthesis and catalysis. Viscosity studies of [C16C16IM][BF4] and the corresponding perchlorate salt demonstrate strong non-Newtonian viscosity behavior for the liquid crystalline state of these ionic liquids.

 Please wait while we load your content...
Please wait while we load your content...