Exploring GPTMS reactivity against simple nucleophiles: chemistry beyond hybrid materials fabrication†
Abstract
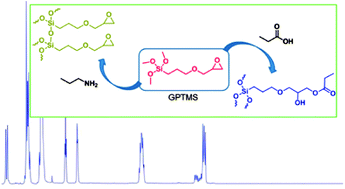
Hybrid materials with interpenetrating networks of silica and degradable polymers have the potential to outperform current biomaterials as their mechanical properties and degradation rates can be tightly controlled. GPTMS is one of the most widely used precursors for sol–gel hybrid fabrication. It can be used as the silica precursor or as a coupling agent to create hybrids with covalent bonds between the silica and organic components. Understanding its reactivity with nucleophilic groups on organic molecules in aqueous conditions is of key importance to hybrid synthesis. NMR spectrometry assisted by mass spectrometry was successfully used for the investigation of the reaction system of (3-glycidoxypropyl)trimethoxysilane (GPTMS) in the presence of different simple nucleophiles. Inorganic condensation and silica network formation is accelerated in the presence of propylamine; propanoic acid gives nucleophilic attack on the epoxy ring; propanol and propanthiol do not seem to participate in the reaction.

 Please wait while we load your content...
Please wait while we load your content...