Copper(i)-catalyzed enantioselective hydroboration of cyclopropenes: facile synthesis of optically active cyclopropylboronates†
Abstract
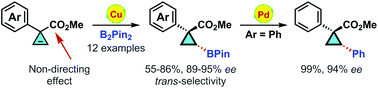
Copper(I)-catalyzed enantioselective hydroboration of 3-aryl substituted cyclopropene-3-carboxylate is described, providing chiral cyclopropylboronates with excellent enantioselectivities (89–95% ee) in moderate to high yields (55–86%). The non-directing effect of the ester group was observed, and the reaction proceeded with solely trans-selectivity. The chiral boronates could be conveniently converted into chiral 1,2-diaryl substituted cyclopropane derivatives.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...