Evaluation of multivalency as an organization principle for the efficient synthesis of doubly and triply threaded amide rotaxanes†
Abstract
Mono-, di- and trivalent pseudorotaxanes with tetralactam macrocycle hosts and axles containing diamide binding stations as the guests have been synthesised. Their threading behaviour was analyzed in detail by NMR experiments and isothermal titration calorimetry. An X-ray crystal structure of the monovalent pseudorotaxane confirms the binding motif. Double mutant cycle analysis provides the effective molarities and insight into the chelate cooperativity of multivalent binding. While the second binding event in a trivalent pseudorotaxane exhibits a slightly positive cooperativity, the third binding is nearly non-cooperative. Nevertheless, the enhanced binding affinities resulting from the multivalent interaction are the basis for a highly efficient synthesis of di- and trivalent rotaxanes through stoppering the axle termini by “click” chemistry. Evidence for the multiply threaded geometry comes from NMR spectroscopy as well as tandem mass-spectrometric fragmentation experiments of mass-selected rotaxane ions in the gas phase. Furthermore, the trivalent rotaxane can be controlled by external stimuli (chloride addition and removal) which lead to an elevator-type movement of the wheel along the axle.
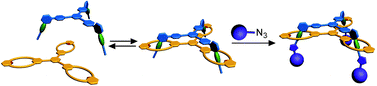

 Please wait while we load your content...
Please wait while we load your content...