New bio-renewable polyester with rich side amino groups from l-lysine via controlled ring-opening polymerization†
Abstract
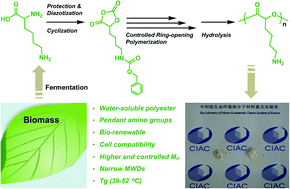
Lysine, a renewable resource from biomass fermentation, was simply converted to its corresponding α-hydroxyl acids and then cyclized to give the pure O-carboxyanhydride (OCA) monomer. Ring-opening polymerization of the resulting monomer was carried out using dimethylaminopyridine (DMAP) as a catalyst in CH2Cl2 at room temperature, and gave well-defined lysine-derived polyesters bearing pendant carbobenzyloxy (Cbz)-protected amino groups with number average molecular weight of up to 45 kg mol−1 in narrow polydispersity. 1H NMR, GPC, and MALDI-TOF MS measurements of the products clearly indicated the controlled/living character of the polymerization. Moreover, amino-functionalized polyesters were readily prepared by the removal of the Cbz protecting group, and the integrity of the polyester backbone was confirmed by 1H NMR. These amino-functionalized polyesters showed a tunable glass transition temperature and exhibited excellent cell compatibility, suggesting their potential to be used as novel materials in biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...