Bis-chlorination of a hexapeptide–PCP conjugate by the halogenase involved in vancomycin biosynthesis†
Abstract
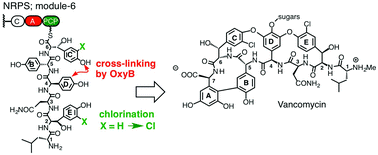
Vancomycin is an important nosocomial antibiotic containing a glycosylated, cross-linked and doubly chlorinated heptapeptide backbone. During the biosynthesis of the vancomycin aglycone, two β-hydroxytyrosine (Bht) residues are inserted at positions-2 and -6 into the heptapeptide backbone by a non-ribosomal peptide synthetase. A single flavin-dependent chlorinase (VhaA) is responsible for chlorinating both Bht residues at some ill-defined point in the assembly process. We show here using in vitro assays that VhaA is able to introduce a chlorine atom into each aromatic ring of both Bht residues at positions-2 and -6 of a peptide carrier protein-bound hexapeptide. The results suggest that VhaA can recognize and chlorinate two quite different sites within a linear hexapeptide intermediate during vancomycin biosynthesis.

 Please wait while we load your content...
Please wait while we load your content...