Regioisomeric allene dimer formation by the reaction of tetraarylbutatrienes with tetracyanoethene†
Abstract
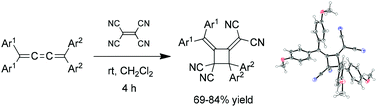
The reaction of tetraarylbutatrienes (tetraaryl[3]cumulenes) with tetracyanoethene (TCNE), a strong electron-accepting molecule, at room temperature yielded novel four-membered ring compounds (head-to-tail unsymmetrically substituted diarylallene dimers) by [2 + 2] cycloaddition of the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the butatrienes. This reaction proceeded through a head-to-head symmetrically substituted diarylallene dimer intermediate. Unsymmetrical butatriene also formed a small amount of another head-to-tail dimer. Although the allene dimer is stable in nonpolar solvents, it converts to other bicyclic and tricyclic compounds in MeOH or CH3CN at room temperature.
C bond of the butatrienes. This reaction proceeded through a head-to-head symmetrically substituted diarylallene dimer intermediate. Unsymmetrical butatriene also formed a small amount of another head-to-tail dimer. Although the allene dimer is stable in nonpolar solvents, it converts to other bicyclic and tricyclic compounds in MeOH or CH3CN at room temperature.

 Please wait while we load your content...
Please wait while we load your content...