ESIPT-induced fluorescent o-hydroxycinnamate: a self-monitoring phototrigger for prompt image-guided uncaging of alcohols†
Abstract
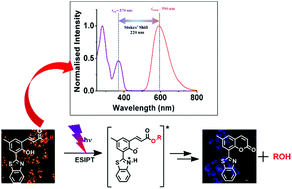
o-Hydroxycinnamate derivatives are well-known phototriggers for fast and direct release of alcohols and amines without proceeding through the cleavage of carbonate or carbamate linkages. Despite these unique features, o-hydroxycinnamates lack extensive applications in biological systems mainly because of their non-fluorescent nature. To overcome this limitation, we have attached a 2-(2′-hydroxyphenyl) benzothiazole (HBT) moiety, capable of rapid excited-state intramolecular proton transfer (ESIPT) to the o-hydroxycinnamate group. The ESIPT effect induced two major advantages to the o-hydroxycinnamate group: (i) large Stokes’ shifted fluorescence (orange colour) properties and (ii) distinct fluorescence colour change upon photorelease. In vitro studies exhibited an image guided, photoregulated release of bioactive molecules by the o-hydroxycinnamate–benzothiazole–methyl salicylate conjugate and real-time monitoring of the release action.


 Please wait while we load your content...
Please wait while we load your content...