Total syntheses of cis-cyclopropane fatty acids: dihydromalvalic acid, dihydrosterculic acid, lactobacillic acid, and 9,10-methylenehexadecanoic acid†
Abstract
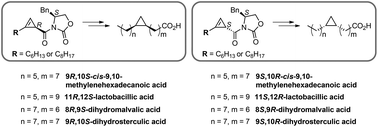
cis-Cyclopropane fatty acids (cis-CFAs) are widespread constituents of the seed oils of subtropical plants, membrane components of bacteria and protozoa, and the fats and phospholipids of animals. We describe a systematic approach to the synthesis of enantiomeric pairs of four cis-CFAs: cis-9,10-methylenehexadecanoic acid, lactobacillic acid, dihydromalvalic acid, and dihydrosterculic acid. The approach commences with Rh2(OAc)4-catalyzed cyclopropenation of 1-octyne and 1-decyne, and hinges on the preparative scale chromatographic resolution of racemic 2-alkylcycloprop-2-ene-1-carboxylic acids using a homochiral Evan's auxiliary. Saturation of the individual diastereomeric N-cycloprop-2-ene-1-carbonylacyloxazolidines, followed by elaboration to alkylcyclopropylmethylsulfones, allowed Julia–Kocienski olefination with various ω-aldehyde-esters. Finally, saponification and diimide reduction afforded the individual cis-CFA enantiomers.

 Please wait while we load your content...
Please wait while we load your content...