Sialylation of lactosyl lipids in membrane microdomains by T. cruzi trans-sialidase†
Abstract
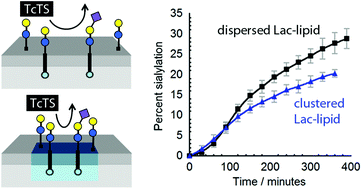
A synthetic perfluoroalkyl-tagged lactosyl glycolipid has been shown to form lipid microdomains in fluid phospholipid bilayers. When embedded in the membranes of phospholipid vesicles, this glycolipid was trans-sialylated by soluble T. cruzi trans-sialidase (TcTS) to give a perfluoroalkyl-tagged glycolipid that displayed the ganglioside GM3 epitope, with up to 35% trans-sialylation from fetuin after 18 h. Following sialylation, vesicles bearing this Neu5Ac(α2-3)Gal(β1-4)Glc sequence in their “glycocalyx” were recognised and agglomerated by the lectin M. amurensis leukoagglutinin. Monitoring TcTS-mediated trans-sialylation by HPLC over the first 6 h revealed that enzymatic transformation of bilayer-embedded substrate was much slower than that of a soluble lactosyl substrate. Furthermore, clustering of the lactose-capped glycolipid into “acceptor” microdomains did not increase the rate of sialic acid transfer from fetuin by soluble TcTS, instead producing slight inhibition.

 Please wait while we load your content...
Please wait while we load your content...