Performance of DFT methods and origin of stereoselectivity in bipyridine N,N′-dioxide catalyzed allylation and propargylation reactions†
Abstract
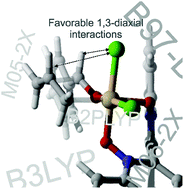
Enantioselectivities for the allylation and propargylation of benzaldehyde catalyzed by bipyridine N,N′-dioxides were predicted using popular DFT methods. The results reveal deficiencies of several DFT methods while also providing a new explanation for the stereoselectivity of these reactions. In particular, even though many DFT methods provide accurate predictions of experimental ee's for these reactions, these predictions sometimes stem from qualitatively incorrect transition states. Overall, B97-D/TZV(2d,2p) provides the best compromise between accurate predictions of low-lying transition states and stereoselectivities for these reactions. The origin of stereoselectivity in these reactions was also examined, and arises from electrostatic interactions within the chiral electrostatic environment of a hexacoordinate silicon intermediate; the previously published transition state model for these reactions is flawed. Ultimately, these results suggest two strategies for the design of highly stereoselective catalysts for the propargylation of aromatic aldehydes, and pave the way for the computational design of novel catalysts for these reactions.

 Please wait while we load your content...
Please wait while we load your content...