Catalytic asymmetric synthesis of the pentacyclic core of (−)-nakadomarin A via oxazolidine as an iminium cation equivalent†
Abstract
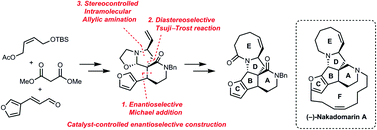
A facile and catalytic asymmetric synthesis of the pentacyclic core of (−)-nakadomarin A, containing all the stereogenic centers of the natural product was achieved. The key intermediate involves the oxazolidine moiety as an iminium cation equivalent. An efficient method for the removal of the N-hydroxyethyl group is also described.

 Please wait while we load your content...
Please wait while we load your content...