Water-soluble NHC-Cu catalysts: applications in click chemistry, bioconjugation and mechanistic analysis†
Abstract
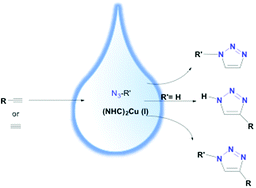
Copper(I)-catalyzed 1,3-dipolar cycloaddition of azides and terminal alkynes (CuAAC), better known as “click” reaction, has triggered the use of 1,2,3-triazoles in bioconjugation, drug discovery, materials science and combinatorial chemistry. Here we report a new series of water-soluble catalysts based on N-heterocyclic carbene (NHC)-Cu complexes which are additionally functionalized with a sulfonate group. The complexes show superior activity towards CuAAC reactions and display a high versatility, enabling the production of triazoles with different substitution patterns. Additionally, successful application of these complexes in bioconjugation using unprotected peptides acting as DNA binding domains was achieved for the first time. Mechanistic insight into the reaction mechanism is obtained by means of state-of-the-art first principles calculations.

 Please wait while we load your content...
Please wait while we load your content...