Synthesis of aromatic 13C/2H-α-ketoacid precursors to be used in selective phenylalanine and tyrosine protein labelling†
Abstract
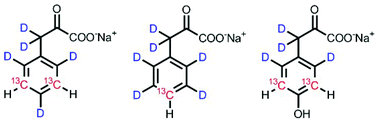
Recent progress in protein NMR spectroscopy revealed aromatic residues to be valuable information sources for performing structure and motion analysis of high molecular weight proteins. However, the applied NMR experiments require tailored isotope labelling patterns in order to regulate spin-relaxation pathways and optimize magnetization transfer. We introduced a methodology to use α-ketoacids as metabolic amino acid precursors in cell-based overexpression of phenylalanine and/or tyrosine labelled proteins in a recent publication, which we have now developed further by providing synthetic routes to access the corresponding side-chain labelled precursors. The target compounds allow for selective introduction of 13C–1H spin systems in a highly deuterated chemical environment and feature alternating 12C–13C–12C ring-patterns. The resulting isotope distribution is especially suited to render straightforward 13C spin relaxation experiments possible, which provide insight into the dynamic properties of the corresponding labelled proteins.

 Please wait while we load your content...
Please wait while we load your content...