Ratiometric fluorescence chemosensor based on tyrosine derivatives for monitoring mercury ions in aqueous solutions†
Abstract
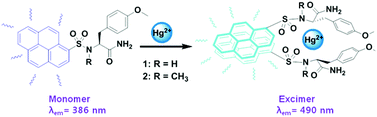
Ratiometric fluorescent chemosensors 1 and 2 were synthesized based on tyrosine amino acid derivatives with a pyrene fluorophore. 1 and 2 showed high selectivity for Hg(II) ions among 13 metal ions in aqueous solutions. Both 1 and 2 sensitively detected Hg(II) ions in aqueous solutions by ratiometric response without interference of any of the other tested metal ions including Cu(II), Cd(II), Pb(II), and Ag(I) ions. 1 and 2 had tight binding affinities (5.72 × 1013 M−2, 1.15 × 1013 M−2) for Hg(II) with nano-molar detection limits. The binding mode was characterized with the help of organic spectroscopic data, which revealed that the methoxyphenyl moieties of 1 and 2 played a vital role in the coordination of Hg(II). The deprotonation of the sulfonamide group is not a critical process for the binding of mercury ions. The methoxyphenyl moiety, sulfonamide group, and the C-terminal amide moiety of 1 and 2 as ligands for Hg(II) played crucial roles in the stabilization of the 2 : 1 complexes.

 Please wait while we load your content...
Please wait while we load your content...