Synthesis of new anionic carbosilane dendrimers via thiol–ene chemistry and their antiviral behaviour†
Abstract
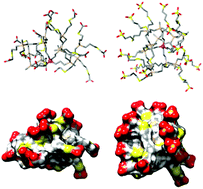
A synthetic strategy has been developed for the preparation of anionic carbosilane dendrimers bearing sulfonate or carboxylate groups at their periphery by means of thiol–ene chemistry. It offers significant advantages, such as milder reaction conditions, shorter reaction times and more facile purification methods, when compared with other synthetic protocols used previously, e.g. hydrosilylation followed by a Michael-type addition or azide–alkyne coupling reactions. Molecular dynamics simulations of the second generation anionic dendrimers addressing shape and size effects of the terminal groups and conformational variability indicated that the core eccentricity and flexibility might need to be taken into account for toxicity and interaction with viral and/or cellular receptors, respectively. The biocompatibility of anionic carbosilane dendrimers has been explored showing differences between silicon-cored and polyphenoxo-cored dendrimers. In addition, silicon-cored dendrimers achieved 85–90% of HIV inhibition without inducing inflammation or vaginal irritation in mice, which makes them likely candidates for readily available, good and safe topical vaginal microbicides against HIV.

 Please wait while we load your content...
Please wait while we load your content...