Synthesis of bicyclic organo-peptide hybrids via oxime/intein-mediated macrocyclization followed by disulfide bond formation†
Abstract
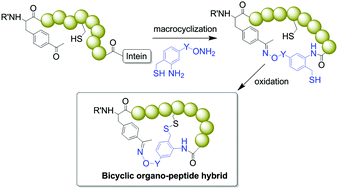
A new strategy is described to generate bicyclic peptides that incorporate non-peptidic backbone elements starting from recombinant polypeptide precursors. These compounds are produced via a one-pot, two-step sequence, in which peptide macrocyclization by means of a bifunctional oxyamine/1,3-amino-thiol synthetic precursor is followed by intramolecular disulfide formation between the synthetic precursor-borne thiol and a cysteine embedded in the peptide sequence. This approach was found to be compatible with the cysteine residue occupying different positions within 8mer and 10mer target peptide sequences and across different synthetic precursor scaffolds, thereby enabling the formation of a variety of diverse bicyclic scaffolds.

 Please wait while we load your content...
Please wait while we load your content...