Formation of a pseudo-β-hairpin motif utilizing the Ant–Pro reverse turn: consequences of stereochemical reordering†
Abstract
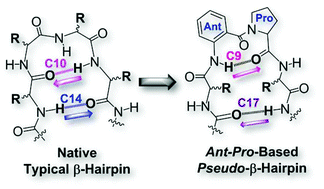
Herein, we report a special case of pseudo-β-hairpin formation by tetrapetide sequences featuring a two-membered Ant–Pro dipeptide motif (Ant = anthranilic acid and Pro = proline) at the loop region. These folded structures uniquely feature the presence of C9- and C17-H-bonding patterns at reverse turn and interstrand regions, respectively. Their hairpin nucleation and folding propensities have been expounded using solution and solid state studies of distinct stereochemically altered sequences.

 Please wait while we load your content...
Please wait while we load your content...