Protein-mediated dethreading of a biotin-functionalised pseudorotaxane†
Abstract
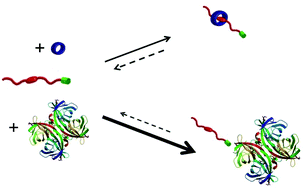
In this article, we describe the synthesis of new biotin-functionalised naphthalene derivatives 3 and 4 and their complexation behaviour with avidin and neutravidin using a range of analytical techniques. We have shown using 2-(4′-hydroxyazobenzene)benzoic acid displacement and ITC experiments, that compounds 3 and 4 have the propensity to form reasonably high-affinity bioconjugates with avidin and neutravidin. We have also demonstrated using 1H NMR, UV-vis and fluorescence spectroscopy that the naphthalene moiety of 3 and 4 facilitates the formation of pseudorotaxane-like structures with 1 in water. We have then investigated the ability of avidin and neutravidin to modulate the complexation between 1 and 3 or 4. UV-vis and fluorescence spectroscopy has shown that in both cases the addition of the protein disrupts complexation between the naphthalene moieties of 3 and 4 with 1.

 Please wait while we load your content...
Please wait while we load your content...