Covalently coupled hybrid of graphitic carbon nitride with reduced graphene oxide as a superior performance lithium-ion battery anode†
Abstract
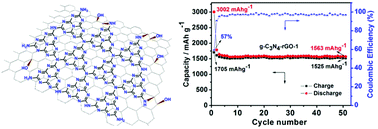
An in situ chemical synthetic approach has been designed for the fabrication of a covalently coupled hybrid consisting of graphitic carbon nitride (g-C3N4) with reduced graphene oxide (rGO) with differing g-C3N4/rGO ratio. The epoxy groups of graphene oxide (GO) undergo a nucleophilic substitution reaction with dicyandiamide (C2H4N4) to form the C2H4N4–GO composite via a covalent C–N bond, and then both the in situ polymerization of C2H4N4 and the thermal reduction of GO can be achieved at higher temperatures, forming the covalently coupled g-C3N4–rGO. FT-IR, CP-MAS NMR and XPS analyses, clearly revealed a covalent interaction between the g-C3N4 and rGO sheets. The g-C3N4–rGO exhibits an unprecedented high, stable and reversible capacity of 1525 mA h g−1 at a current density of 100 mA g−1 after 50 cycles. Even at a large current density of 1000 mA g−1, a reversible capacity of 943 mA h g−1 can still be retained. The superior electrochemical performance of g-C3N4–rGO is attributed to the specific characteristics of the unique nanostructure of g-C3N4–rGO and the concerted effects of g-C3N4 and rGO, including covalent interactions between the two moieties, the good conductivity and high special surface area of the nanocomposite, as well as the template effect of the planar amino group of g-C3N4 for the dispersed decoration of Li+ ions.

 Please wait while we load your content...
Please wait while we load your content...