Driving forces for the self-assembly of graphene oxide on organic monolayers†
Abstract
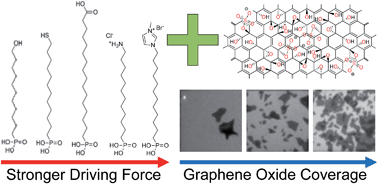
Graphene oxide (GO) flakes were self-assembled from solution on surfaces of self-assembled monolayers (SAMs), varying in the chemical structure of their head groups. The coverage density of GO relates to strength of attractive interaction, which is largest for Coulomb interaction provided by positively charged SAM head groups and negatively charged GO. A rough surface enhances the coverage density but with the same trend in driving force dependency. The self-assembly approach was used to fabricate field-effect transistors with reduced GO (rGO) as active layer. The SAMs as attractive layer for self-assembly remain almost unaffected by the reduction from GO to rGO and serve as ultra-thin gate dielectrics in devices, which operate at low voltages of maximum 3 V and exhibit a shift of the Dirac voltage related to the dipole moment of the SAMs.

 Please wait while we load your content...
Please wait while we load your content...