Water and methanol adsorption on MOFs for cycling heat transformation processes†
Abstract
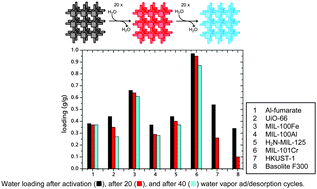
Microporous materials with high water uptake capacity are gaining attention for low temperature heat transformation applications such as thermally driven adsorption chillers (TDCs) or adsorption heat pumps (AHPs). TDCs or AHPs are alternatives to traditional air conditioners or heat pumps operating on electricity or fossil fuels. By using solar or waste heat as the driving energy, TDCs or AHPs can minimize primary energy consumption. TDCs and AHPs are based on the evaporation and consecutive adsorption of coolant liquids, preferably water, under specific conditions. Their ranges of application, as well as their efficiencies, power densities and total costs, are substantially influenced by the microporosity and hydrophilicity of the employed sorption materials. Here, we briefly summarize current investigations, developments and possibilities of metal–organic frameworks (MOFs) compared to classical materials. With their high water uptake, MOFs surpass those materials, while, at the same time, the variability of the building blocks allows for tuning of the microporosity and hydrophobic/hydrophilic design, depending on the specific application.
- This article is part of the themed collection: Advanced Complex Inorganic Nanomaterials

 Please wait while we load your content...
Please wait while we load your content...