Green and easily scalable microwave synthesis of noble metal nanosols (Au, Ag, Cu, Pd) usable as catalysts
Abstract
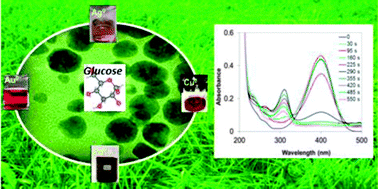
A green synthesis process was developed for the production of PVP-coated noble metal nanoparticles in the form of stable nanosols. Water is the environmentally benign solvent; glucose serves as a mild, renewable and non-toxic reducing agent and microwave irradiation is an effective and fast heating technique. The same green process has been optimized to obtain several metal nanoparticles (Au, Ag, Cu, Pd), and therefore encourages the easy preparation of bimetallic nanostructures. Nanosols were characterized by dynamic light scattering DLS, HR-TEM, UV-Vis spectroscopy, XRD and ICP-AES. The total reaction yield for all the samples was assessed, the prepared nanoparticles were spherical shaped with an average diameter ranging from 3 to 20 nm. Nanosols with excellent stability over several months, achieved even for high solid contents, were prepared. Additionally, it is shown that all of the synthesized nanoparticles can act as effective catalysts for the reduction of 4-nitrophenol (4-NP) in the presence of NaBH4 (which is otherwise unfeasible without a metal catalyst). This reduction was spectrophotocally followed and the rate constants were determined by measuring the change in absorbance at 400 nm (the wavelength typical of 4-NP) as a function of time. The following ranking of decreasing efficiency of the catalyst was found: Pd > Au > Ag > Cu.

 Please wait while we load your content...
Please wait while we load your content...