Branched α-d-mannopyranosides: a new class of potent FimH antagonists†
Abstract
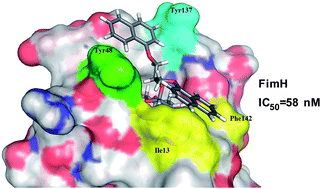
FimH is a type I fimbrial lectin located at the tip of type-1 pili of uropathogenic Escherichia coli guiding its ability to adhere and infect urothelial cells. Accordingly, blocking FimH with small-molecule antagonists is considered as a promising new therapeutic alternative to treat infections caused by uropathogenic Escherichia coli (UPEC). Herein we report that our recently disclosed α-D-mannopyranosides bearing diaryl substituted 1,3-diaminopropanol or glycerol moieties act as potent FimH antagonists in vitro, as determined by a competitive binding assay on isolated FimH lectin. Most of the assayed compounds display FimH antagonistic activity in the range of 58–1000 nM. Based on the promising results of the first series of compounds, we have designed and synthesized a new series of asymmetrically disubstituted glyceryl α-D-mannopyranosides with improved physicochemical properties. Molecular docking calculations were employed to predict compounds' binding poses leading to two possible binding modes; so-called in- and out-docking modes in the “tyrosine gate” (formed by Tyr48 and Tyr137) for one aromatic moiety, which is in accordance with previous findings, while the second aromatic moiety reaches the previously unexplored lipophilic region formed by Phe142 and Ile13. Furthermore, compounds were found to be non-cytotoxic on HepG2 cells in concentrations up to 10 μM pointing to their selective toxicity, which is one of the key features of potential therapeutics for the treatment of urinary tract infections.

 Please wait while we load your content...
Please wait while we load your content...