Identification of an inhibitor of the ubiquitin–proteasome system that induces accumulation of polyubiquitinated proteins in the absence of blocking of proteasome function
Abstract
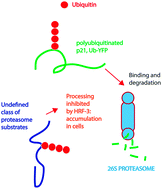
The ubiquitin–proteasome system (UPS) represents one of the most promising therapeutic targets in oncology to emerge in recent years. Here we used a combination of cytotoxic and image-based screening assays to identify a novel UPS inhibitor, designated HRF-3. HRF-3 evokes a gene expression profile similar to that of other characterized UPS inhibitors, suggesting a common mechanism of action. Consistent with UPS inhibition, HRF-3 induced strong accumulation of polyubiquitinated proteins in cells. Surprisingly, HRF-3 induced only weak accumulation of two proteasome targeted reporter proteins, UbG76V-YFP and ZsGreen-ODC. Consistent with this observation, HRF-3 did not inhibit proteasome proteolytic activity in an in vitro assay. Similar to a number of other UPS inhibitors, HRF-3 increased the expression of the redox-inducible protein Hmox-1. In distinction to the 20S inhibitor bortezomib, but similarly to two different p97/VCP inhibitors, HRF-3 did not elicit strong induction of the chaperone Hsp70B′. Finally, we show that HRF-3 is cytotoxic to a variety of cancer cell lines and ex vivo patient tumour cells, with the strongest activity observed in cells of leukemic/myeloma origin. Taken together our data show that HRF-3 induces polyubiquitin accumulation in the absence of efficient proteasomal blocking, and suggest that induction of oxidative stress is a common denominator of UPS inhibitors.

 Please wait while we load your content...
Please wait while we load your content...