Excitation of higher levels of singly charged copper ions in argon and neon glow discharges
Abstract
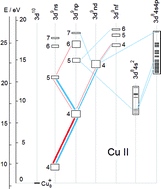
Transition rate diagrams of copper ions in argon and neon glow discharges are presented, using data from Cu II emission spectra. Based on the transition rate diagrams, several different collisional processes between ground state and metastable atoms and ions of copper and the discharge gas are proposed as probably significant for populating upper levels of the Cu II emission lines observed.
- This article is part of the themed collection: JAAS Emerging Investigator Lectureship winners

 Please wait while we load your content...
Please wait while we load your content...