Cleaving C–H bonds with hyperthermal H2: facile chemistry to cross-link organic molecules under low chemical- and energy-loads†
Abstract
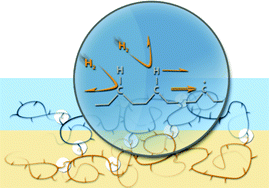
A facile method for cross-linking organic molecules has been developed by computational modeling, instrumentation design, and experimental research. Briefly, organic molecules are hit by H2 with controllable kinetic energy in our novel apparatus where a high flux of hyperthermal H2 is generated. When a C–H bond of the organic molecule is hit by H2 at about 20 eV, efficient kinematic energy-transfer in the H2→H collision facilitates the C–H dissociation with nearly 100% reaction probability. When H2 hits other atoms which are by nature much heavier than H2, mass disparity bars effective energy transfer and this both blocks undesirable bond dissociation and reduces unnecessary energy wastage. The recombination of the carbon radicals generated by the C–H cleavage efficiently completes the production of C–C cross-links at room temperature with no additional energy/chemicals requirements. In addition to these green chemistry merits, this new method is better than other cross-linking techniques which rely on prerequisite reactions to add cross-linkers to the organic molecules or additional reactants and additives. These promising features are validated by several cross-linking trials which demonstrate desirable mechanical, electrical, chemical, and biochemical changes while inducing no undesirable damage of chemical functionalities in the original molecules.

 Please wait while we load your content...
Please wait while we load your content...