The in situ transformation of the co-product formaldehyde in the reversible hydrolysis of 1,3-dixoane to obtain 1,3-propanediol efficiently†
Abstract
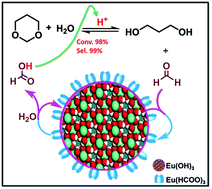
Herein, a strategy is developed for efficient production of l,3-propanediol via the hydrolysis of 1,3-dioxane by the in situ transformation of the co-product formaldehyde (HCHO) in the presence of Eu(OH)3. The reversible hydrolysis reaction is promoted to yield 98% conversion and 99% 1,3-propanediol selectivity. Furthermore, HCHO is converted to formic acid (HCOOH) which could act as an acidic catalyst in the hydrolysis of 1,3-dioxane. The combination of FT-IR and control experiments demonstrates that HCOOH is generated via the hydrolysis of formate species which formed on the surface of Eu(OH)3.


 Please wait while we load your content...
Please wait while we load your content...