Visible light organophotoredox catalysis: a general approach to β-keto sulfoxidation of alkenes†
Abstract
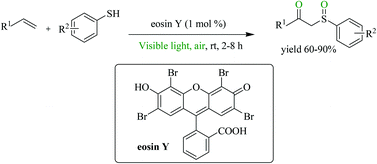
A mild, simple, efficient and metal-free approach to β-keto sulfoxides utilising eosin Y as an organophotoredox catalyst has been developed. This general protocol offers a direct and rapid difunctionalization of alkenes using thiophenols and atmospheric oxygen at room temperature in a one-pot procedure. The utilisation of visible light and air (O2) as inexpensive, readily available, non-toxic and eco-sustainable reagents makes this protocol compatible with the green chemistry demands.

 Please wait while we load your content...
Please wait while we load your content...