Magnetic gold nanocatalyst (nanocat-Fe–Au): catalytic applications for the oxidative esterification and hydrogen transfer reactions†
Abstract
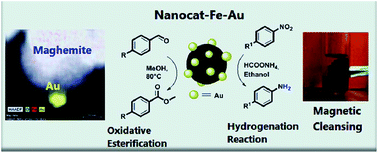
An efficient and sustainable protocol is described for the oxidative esterification of aldehydes and the reduction of aromatic nitro compounds that uses a magnetically separable and reusable maghemite-supported gold nanocatalyst (nanocat-Fe–Au) under mild conditions. The complex chemical, morphological, structural and size analyses, including e.g. XPS, HRTEM, in-field Mössbauer spectroscopy and HRTEM, revealed that the hybrid material is composed of a well-defined stoichiometric maghemite support (20–30 nm) decorated with ultrasmall (5–6 nm) gold nanoparticles. The hybrid catalytic system containing 4 wt% of nanogold has been generated using simple impregnation methods in aqueous medium from readily available starting materials and was recycled five times without any significant loss in catalytic activity; high yields, 40–95% and 83–94% for oxidative esterification and reduction reactions, respectively, were obtained.

 Please wait while we load your content...
Please wait while we load your content...