Palladium nanoparticles supported on triazine functionalised mesoporous covalent organic polymers as efficient catalysts for Mizoroki–Heck cross coupling reaction†
Abstract
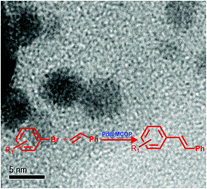
A novel class of mesoporous covalent organic polymer (MCOP) was synthesised by the nucleophilic substitution of cyanuric chloride with 4,4′-dihydroxybiphenyl. The MCOP was fully characterized using powder X-ray diffraction analysis, Fourier transform infrared spectroscopy, 13C-solid state NMR spectroscopy, field emission scanning electron microscopy and thermogravimetric analysis. These nitrogen rich materials act as good supports for palladium nanoparticles (Pd NPs) and exhibit excellent catalytic activity towards Mizoroki–Heck cross coupling between aryl bromides and alkenes. Hot filtration tests demonstrate that the presence of the triazine rings on the polymers is beneficial for enhancing the stability of Pd NPs. The polymers are also cheap, easy to synthesise and can be recycled up to five times with only a minor loss of activity.

 Please wait while we load your content...
Please wait while we load your content...