Vanillin, a promising biobased building-block for monomer synthesis
Abstract
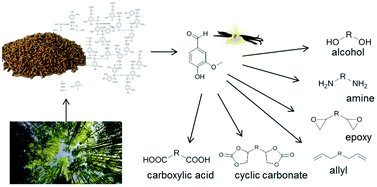
Vanillin was used as a renewable building-block to develop a platform of 22 biobased compounds for polymer chemistry. Vanillin-derived biobased monomers bearing epoxy, cyclic carbonates, allyl, amine, alcohol and carboxylic acid moieties were synthesized. They can be used, among many others, in epoxy, polyester, polyurethanes, and Non-Isocyanate PolyUrethanes (NIPU) polymer synthesis. The epoxy-functionalized compounds were synthesized under solvent-free conditions and are original biobased aromatic epoxy monomers. Cyclic carbonates were prepared through a catalytic reaction between epoxy compounds and CO2. Thiol–ene reactions allowed the functionalization of allylated compounds with amines, acids and alcohols. The amine-functionalized compounds are, to our knowledge, the first non-aliphatic biobased amine hardeners, usable either in epoxy or NIPU materials.

 Please wait while we load your content...
Please wait while we load your content...