Influence of different types of natural organic matter on titania nanoparticle stability: effects of counter ion concentration and pH†
Abstract
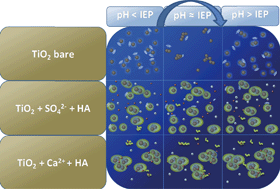
The effects of biopolymeric (alginate) and refractory macromolecules (humic and fulvic acids) on the aggregation kinetics of anatase titanium dioxide (titania) nanoparticles were evaluated. The particles were synthesized using a wet-chemical method based on the hydrolysis of TiCl4. Stable suspensions of positively-charged titania were obtained at pH 2.5. One batch of this product was shifted above the point of zero charge to pH 12. These dispersions were mixed with three different types of well characterized environmental macromolecules: sodium alginate, fulvic acid and humic acid, and evaluated in terms of the stability. Changes in the particle size were measured using time-resolved dynamic light scattering (TR-DLS) at different concentrations of three electrolytes: NaCl, CaCl2 and Na2SO4, and at different solution pH values. Results were in agreement with DLVO calculations. The use of TR-DLS for determining aggregation rates is critically discussed.

 Please wait while we load your content...
Please wait while we load your content...