Lithium–sulfur batteries—the solution is in the electrolyte, but is the electrolyte a solution?
Abstract
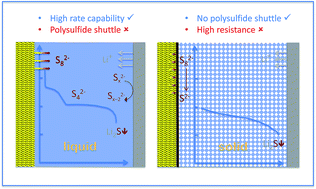
At first glance, the combination of the lightest, most electropositive metal (lithium) with a safe, abundant (and reasonably light) non-metal (sulfur) makes good sense as a prospective battery. However, while the lithium–sulfur battery offers a very high theoretical specific energy (∼2600 W h kg−1) the actual performance delivered is proving to be severely limited—in many cases, this is directly related to the role of the electrolyte. The fundamental issue is that the reduction of sulfur proceeds through a series of polysulfide species, which are for the most part soluble in common organic solvents, including those employed in battery electrolyte solutions. So, despite the fact that the ultimate product (Li2S) is essentially insoluble, the intermediate stages of discharge see a migration of redox-active species out of the cathode, from where they can react with the lithium anode, which sets in train a series of equilibria that cause both a loss of charging efficiency and a gradual loss of discharge capacity. In the last decade, a major stream of the research to overcome this complex situation has focused on minimizing the solubility of polysulfides. From this we now have a range of media in which the lithium–sulfur system can operate with much improved charge–discharge characteristics: ionic liquids (and blends with organic media); super-saturated salt-solvent mixtures; polymer-gelled organic media; solid polymers; solid inorganic glasses. Underlining the multi-faceted nature of interactions within the lithium–sulfur cell, though, none of these improved electrolytes has been able to bring the performance of this system up to the levels of reliability and capacity maintenance (without sacrificing high specific energy) that are benchmarks in energy storage applications. Our survey indicates that only by combining particular electrolytes with cathode materials that are designed to actively retain sulfur and its reduction products, have a relatively few studies been able to obtain the desired levels of performance. Ultimately the successful development of the lithium–sulfur battery requires careful coordination of the choice of modified electrolyte with the specific nature of the cathode material, underpinned by the assumption that the resulting electrolyte composition will meet established criteria for compatibility with the lithium anode.

 Please wait while we load your content...
Please wait while we load your content...