A super-efficient cobalt catalyst for electrochemical hydrogen production from neutral water with 80 mV overpotential†
Abstract
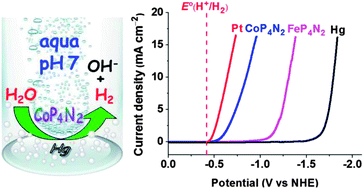
Self-assembled molecular iron and cobalt catalysts (MP4N2, M = Fe, Co) bearing a multihydroxy-functionalized tetraphosphine ligand electrocatalyze H2 generation from neutral water on a mercury electrode at −1.03 and −0.50 V vs. NHE, respectively. Complex CoP4N2 displays extremely low overpotential (Eonset = 80 mV) while maintaining high activity and good stability. Bulk electrolysis of CoP4N2 in a neutral phosphate buffer solution at −1.0 V vs. NHE produced 9.24 × 104 mol H2 per mol cat. over 20 h, with a Faradaic efficiency close to 100% and without apparent deactivation.

 Please wait while we load your content...
Please wait while we load your content...