Rhodafuran from a methoxy(alkenyl)carbene by the rhoda-1,3,5-hexatriene route†
Abstract
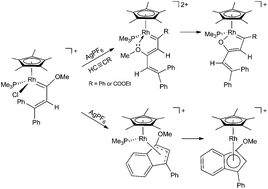
A methoxy(alkenyl)carbenerhodium complex [RhCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)CH
C(OMe)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2}(PMe3)]PF6 (2) has been synthesized and used as the starting material for the study of the effect of the metal center (Rh vs. Ir) in the formation of new rhodacycle complexes. While η3 and η5 indenylrhodium complexes have been achieved by the C–H bond activation of a phenyl ring, insertion of terminal alkynes into the rhodium–carbene bond led to the first example of the synthesis of rhodafuran complexes through rhoda-1,3,5-hexatriene intermediates. This new method represents an efficient process to obtain metallafuran complexes.
CPh2}(PMe3)]PF6 (2) has been synthesized and used as the starting material for the study of the effect of the metal center (Rh vs. Ir) in the formation of new rhodacycle complexes. While η3 and η5 indenylrhodium complexes have been achieved by the C–H bond activation of a phenyl ring, insertion of terminal alkynes into the rhodium–carbene bond led to the first example of the synthesis of rhodafuran complexes through rhoda-1,3,5-hexatriene intermediates. This new method represents an efficient process to obtain metallafuran complexes.


 Please wait while we load your content...
Please wait while we load your content...