Excited state evolution towards ligand loss and ligand chelation at group 6 metal carbonyl centres†
Abstract
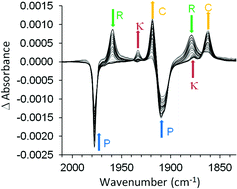
The photochemistry and photophysics of three model “half-sandwich” complexes (η6-benzophenone)Cr(CO)3, (η6-styrene)Cr(CO)3, and (η6-allylbenzene)Cr(CO)3 were investigated using pico-second time-resolved infrared spectroscopy and time-dependent density functional theory methods. The (η6-benzophenone)Cr(CO)3 complex was studied using two excitation wavelengths (470 and 320 nm) while the remaining complexes were irradiated using 400 nm light. Two independent excited states were detected spectroscopically for each complex, one an unreactive excited state of metal-to-arene charge-transfer character and the other with metal-to-carbonyl charge transfer character. This second excited state leads to an arrested release of CO on the pico-second time-scale. Low-energy excitation (470 nm) of (η6-benzophenone)Cr(CO)3 populated only the unreactive excited state which simply relaxes to the parent complex. Higher energy irradiation (320 nm) induced CO-loss. Irradiation of (η6-styrene)Cr(CO)3, or (η6-allylbenzene)Cr(CO)3 at 400 nm provided evidence for the simultaneous population of both the reactive and unreactive excited states. The efficiency at which the unreactive excited state is populated depends on the degree of conjugation of the substituent with the arene π-system and this affects the efficiency of the CO-loss process. The quantum yield of CO-loss is 0.50 for (η6-allylbenzene)Cr(CO)3 and 0.43 for (η6-styrene)Cr(CO)3. These studies provide evidence for the existence of two photophysical routes to CO loss, a minor ultrafast route and an arrested mechanism involving the intermediate population of a reactive excited state. This reactive excited state either relaxes to reform the parent species or eject CO. Thus the quantum yield of the CO-loss is strongly dependent on the excitation wavelength. Time-dependent density functional theory calculations confirm that the state responsible for ultrafast CO-loss has significant metal-centred character while the reactive state responsible for the arrested CO-loss has significant metal-to-carbonyl charge-transfer character. The CO-loss product (η6-allylbenzene)Cr(CO)2 formed following irradiation of (η6-allylbenzene)Cr(CO)3 reacts further with the pendent alkenyl group to form the chelate product (η6,η2-allylbenzene)Cr(CO)2.
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States

 Please wait while we load your content...
Please wait while we load your content...