Variation of electronic transitions and reduction potentials of cerium(iv) complexes†
Abstract
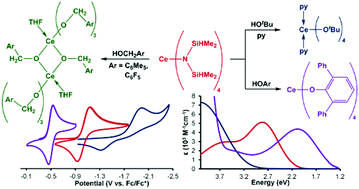
The trivalent compound K[Ce[N(SiHMe2)2]4] was synthesized and oxidized, providing a convenient route to the reported cerium(IV) compound Ce[N(SiHMe2)2]4. Protonolysis reactions of Ce[N(SiHMe2)2]4 with tert-butanol, substituted benzyl alcohols, and 2,6-diphenylphenol yielded the neutral tetravalent compounds Ce(OtBu)4(py)2, Ce2(OCH2C6R5)8(thf)2 (R = Me, F), and Ce(Odpp)4 (dpp = 2,6-(C6H5)2-C6H3). Spectroscopic and electrochemical characterization of the monometallic cerium(IV) silylamide, alkoxide, and aryloxide compounds revealed variable ligand-to-metal charge transfer transitions and metal-based reduction potentials. Computational bonding analyses were performed to complement the physical characterization of the complexes.

 Please wait while we load your content...
Please wait while we load your content...