The important role of the Mo–Mo quintuple bond in catalytic synthesis of benzene from alkynes. A theoretical study†
Abstract
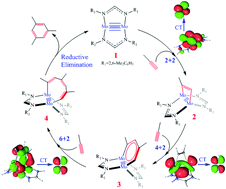
The Mo–Mo quintuple bond was recently applied to catalytic synthesis of benzene from alkynes, which is the first example of the catalytic reaction of the metal–metal multiple bond. This new reaction was studied using DFT and CASSCF/CASPT2 methods. The entire catalytic cycle consists of four steps: [2 + 2], [4 + 2], and [6 + 2] cycloadditions, and reductive elimination of benzene. The symmetry-forbidden [2 + 2] cycloaddition and asymmetric [2 + 2] cycloaddition are two possible pathways for the reaction between an alkyne and the Mo–Mo quintuple bond. Though the barrier of the former pathway is moderate because of the presence of the multi-reference character of the Mo–Mo quintuple bond, the asymmetric pathway is much more favorable because of its symmetry-allowed feature. The C–C bond formation in the next [4 + 2] cycloaddition occurs through charge transfer (CT) from the π orbital of the incoming alkyne to the π* orbital of another alkyne coordinating with the Mo center to afford a novel dimolybdenacyclic species 3. In 3, the δdxz and 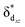 orbitals of the Mo–Mo moiety and four π orbitals of the [C4H4] moiety construct the π and π* orbitals in the six-membered ring. The next [6 + 2] cycloaddition between 3 and one more alkyne affords an eight-membered ring compound 4 which has a Mo–Mo quadruple bond. This is the rate-determining step of the entire catalytic cycle, the ΔG0‡ value of which is 22.4 kcal mol−1. The subsequent reductive elimination of benzene easily occurs to yield a μ2–η2:η2-benzene dinuclear Mo complex with a Mo–Mo quintuple bond. On the other hand, further [8 + 2] cycloaddition between 4 and one more alkyne is much more unfavorable than the reductive elimination of benzene. The similar [4 + 2] process between alkyne and a Cr–Cr quadruple bond is calculated to be difficult, which is consistent with the experimental result that only the Mo–Mo quintuple bond was successfully applied to this reaction. It is likely that the crowded coordination environment and the much more stable πdyz orbital in the Cr–Cr quadruple bond are responsible for the difficulty in the reaction.
orbitals of the Mo–Mo moiety and four π orbitals of the [C4H4] moiety construct the π and π* orbitals in the six-membered ring. The next [6 + 2] cycloaddition between 3 and one more alkyne affords an eight-membered ring compound 4 which has a Mo–Mo quadruple bond. This is the rate-determining step of the entire catalytic cycle, the ΔG0‡ value of which is 22.4 kcal mol−1. The subsequent reductive elimination of benzene easily occurs to yield a μ2–η2:η2-benzene dinuclear Mo complex with a Mo–Mo quintuple bond. On the other hand, further [8 + 2] cycloaddition between 4 and one more alkyne is much more unfavorable than the reductive elimination of benzene. The similar [4 + 2] process between alkyne and a Cr–Cr quadruple bond is calculated to be difficult, which is consistent with the experimental result that only the Mo–Mo quintuple bond was successfully applied to this reaction. It is likely that the crowded coordination environment and the much more stable πdyz orbital in the Cr–Cr quadruple bond are responsible for the difficulty in the reaction.

 Please wait while we load your content...
Please wait while we load your content...