Iron(ii) complexes of ditopic carbanionic carbenes†
Abstract
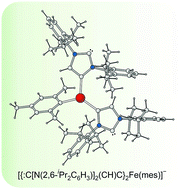
Reaction of dimesityliron(II) (Fe2(mes)4) with the N-heterocyclic carbenes 1,3-bis(2,6-diisopropylphenyl)-imidazol-2-ylidene (IPr) and 1,3-bis(2,6-dimethylphenyl)hexahydropyrimidin-2-ylidene (6-Xyl) afforded the novel trigonal planar complexes [Fe(IPr)(mes)2] (1) and [Fe(6-Xyl)(mes)2] (2), respectively. Both species were structurally characterized by single crystal X-ray diffraction and display structures and magnetic responses consistent with a quintet ground state (S = 2). Reaction of 1 with KC8 in THF afforded K+ salts of the anionic complex [{:C[N(2,6-iPr2C6H3)]2(CH)C}2Fe(mes)]− (3) and the homoleptic organometallic anion [Fe(mes)3]− (4). By contrast, reduction of 2 resulted in extensive decomposition and intractable product mixtures. Complex 3 is coordinated by two ditopic carbanionic carbenes via the C4/C5 position while the C2 position retains unquenched carbenic character and remains vacant for further coordination. This was corroborated by reacting solutions of 3 with one and two equivalents of triethylaluminium (AlEt3) which resulted in the formation of [{Et3Al:C[N(2,6-iPr2C6H3)]2(CH)C}{:C[N(2,6-iPr2C6H3)]2(CH)C}Fe(mes)]− (5) and [{Et3Al:C[N(2,6-iPr2C6H3)]2(CH)C}2Fe(mes)]− (6), respectively. Both of these species were structurally characterized as [K(2,2,2-crypt)]+ salts.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...