Bismuth(iii) β-thioxoketonates as antibiotics against Helicobacter pylori and as anti-leishmanial agents†
Abstract
Nine different β-thioxoketones of general formula R1C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CH2C(
O)CH2C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)R2 (R1 = C6H5, R2 = C6H5L1; R1 = C6H5, R2 = p-CF3C6H4L2; R1 = p-MeOC6H4, R2 = C6H5L3; R1 = p-MeOC6H4, R2 = p-CF3C6H4L4; R1 = C5H4N, R2 = C6H5L5; R1 = p-IC6H4, R2 = C6H5L6; R1 = C6H5, R2 = p-IC6H4L7; R1 = C6H5, R2 = C10H7L8 and R1 = CH3, R2 = C6H5L9) and their tris-substituted bismuth(III) complexes having the general formula [Bi{R1C(
S)R2 (R1 = C6H5, R2 = C6H5L1; R1 = C6H5, R2 = p-CF3C6H4L2; R1 = p-MeOC6H4, R2 = C6H5L3; R1 = p-MeOC6H4, R2 = p-CF3C6H4L4; R1 = C5H4N, R2 = C6H5L5; R1 = p-IC6H4, R2 = C6H5L6; R1 = C6H5, R2 = p-IC6H4L7; R1 = C6H5, R2 = C10H7L8 and R1 = CH3, R2 = C6H5L9) and their tris-substituted bismuth(III) complexes having the general formula [Bi{R1C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CHC(
O)CHC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)R2}3] were synthesised and fully characterised. The solid state structure of [Bi{C5H4NC(
S)R2}3] were synthesised and fully characterised. The solid state structure of [Bi{C5H4NC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CHC(
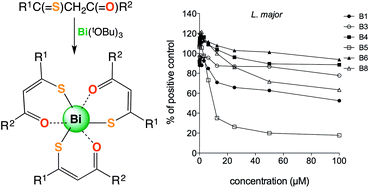
O)CHC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)C6H5}3] B5 was determined by crystallography and revealed that the three β-thioxoketonato ligands are bound to bismuth(III) centre in a bidentate fashion through O and S atoms. The bismuth(III) complexes and the corresponding thioxoketones were assessed for their activity against H. pylori. All of the bismuth(III) complexes were highly active against H. pylori having a MIC of greater than or equal to 3.125 μg mL−1, while the free acids were essentially not toxic to the bacteria. The anti-leishmanial activity of all the bismuth(III) β-thioxoketonates and the corresponding free acids were assessed against L. major promastigotes. The toxicity towards human fibroblast cells was also assessed. All of the free β-thioxoketones were selectively toxic to the L. major promastigotes displaying some potential as anti-leishmanial agents. Among these [C6H5C(
S)C6H5}3] B5 was determined by crystallography and revealed that the three β-thioxoketonato ligands are bound to bismuth(III) centre in a bidentate fashion through O and S atoms. The bismuth(III) complexes and the corresponding thioxoketones were assessed for their activity against H. pylori. All of the bismuth(III) complexes were highly active against H. pylori having a MIC of greater than or equal to 3.125 μg mL−1, while the free acids were essentially not toxic to the bacteria. The anti-leishmanial activity of all the bismuth(III) β-thioxoketonates and the corresponding free acids were assessed against L. major promastigotes. The toxicity towards human fibroblast cells was also assessed. All of the free β-thioxoketones were selectively toxic to the L. major promastigotes displaying some potential as anti-leishmanial agents. Among these [C6H5C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CH2C(
O)CH2C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)C6H5] L1 and [C5H4NC(
S)C6H5] L1 and [C5H4NC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CH2C(
O)CH2C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)C6H5] L5 showed comparable activity to that of Amphotericin B, killing about 80% of the L. major promastigotes at a concentration of 25 μM (6.0 μg mL−1). The bismuth(III) β-thioxoketonate complexes were toxic to both the L. major promastigotes and fibroblast cells at high concentrations, but gave no improvement in anti-leishmanial activity over the free β-thioxoketones.
S)C6H5] L5 showed comparable activity to that of Amphotericin B, killing about 80% of the L. major promastigotes at a concentration of 25 μM (6.0 μg mL−1). The bismuth(III) β-thioxoketonate complexes were toxic to both the L. major promastigotes and fibroblast cells at high concentrations, but gave no improvement in anti-leishmanial activity over the free β-thioxoketones.

 Please wait while we load your content...
Please wait while we load your content...