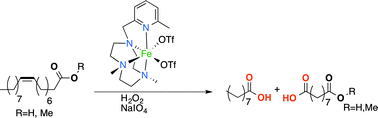
Fe(6-Me-PyTACN)-catalyzed, one-pot oxidative cleavage of methyl oleate and oleic acid into carboxylic acids with H2O2 and NaIO4†
Abstract
The first Fe-based catalytic system for the oxidative cleavage of unsaturated fatty acids and esters to carboxylic acids is reported. The system comprises [Fe(OTf)2(6-Me-PyTACN)] (2) (6-Me-PyTACN = 1-[(6-methyl-2-pyridyl)methyl]-4,7-dimethyl-1,4,7-triazacyclononane, OTf = trifluoromethane sulfonate anion) as the catalyst (3 mol%) either with a combination of hydrogen peroxide and NaIO4 or exclusively with NaIO4 as the oxidant, and operates at 0 °C or ambient temperature. Under these standard conditions (method A), methyl oleate is converted in a one-pot procedure into 50–55% of both nonanoic and azelaic acid, together with some epoxide and aldehyde intermediates as byproducts. These yields can be further improved by addition of sulfuric acid (method B) to hydrolyze the epoxide byproducts, by including a pH neutralization step and addition of more catalyst (1 mol%). Under the optimized conditions, both methyl oleate and oleic acid are converted into high yields of the corresponding carboxylic acids (80–85%). Overall, this catalytic system provides an alternative to the industrial ozonolysis of oleic acid and to catalytic Ru- and Os-based systems for the oxidative cleavage of unsaturated fatty acids and esters.

 Please wait while we load your content...
Please wait while we load your content...