A survey of pendant donor-functionalised (N,O) phosphine ligands for Cr-catalysed ethylene tri- and tetramerisation†
Abstract
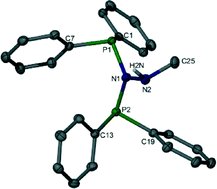
In this study three classes of ligands are explored for ethylene tri-/tetramerisation in conjunction with chromium and both triethylaluminium (AlEt3) and methylaluminoxane (MAO) co-catalysts. Hydrazine based ligands containing an N–H functionality [PN(NH)P], analogous to Rosenthal's previously reported PNPNH system, show selectivity towards 1-hexene and 1-octene formation in conjunction with AlEt3, and act as PNP tetramerisation analogues when MAO is employed. PNP ligands containing non-protic pendant donor moieties generally show poor activity and selectivity when AlEt3 is employed as an activator, however when MAO is used good activities and selectivities are achieved. n-Propylcyclopentane and 2-propenylcyclopentane, and higher homologues, are produced during catalysis when oxygen is the donor atom. The formation of such products is discussed with respect to the generation of methylenecyclopentane and methylcyclopentane by PNP based tetramerisation catalysts. Simple phosphine ligands containing O–H functionalisation are also explored and it was shown that the catalyst selectivity is highly dependent on both the activator and structural features of the ligand employed.

 Please wait while we load your content...
Please wait while we load your content...