Bi-aryl rotation in phenyl-dihydroimidazoquinoline catalysts for kinetic resolution of arylalkyl carbinols†
Abstract
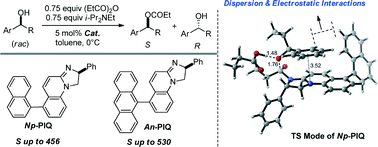
Chiral nucleophilic catalysts, 6-aryl-phenyl-dihydroimidazoquinolines (PIQs), were designed, synthesised and applied to the kinetic resolution of arylalkyl carbinols with very high selectivity (S) factors (up to 530). Density functional theory calculations indicate that multiple noncovalent interactions play a key role in chiral recognition between 6-aryl-PIQ catalysts and chiral secondary alcohol substrates.

 Please wait while we load your content...
Please wait while we load your content...