Structure and properties of bimetallic titanium and vanadium oxide clusters
Abstract
By employing a genetic algorithm together with density functional theory (B3LYP), we investigate the most stable minimum structures of several bimetallic titanium and vanadium oxide clusters that contain four metal atoms. The following compositions are studied: VnTin−4O10− (n = 1–4), (TiO2)VOn− (n = 1–4), and (TiO2)VOn+ (n = 1–3). Apart from (TiO2)3VO−, vanadium oxo groups are always part of the most stable minimum structures when vanadium is present. Anti-ferromagnetic coupling lowers the energy substantially if spin centers are located at neighbored metal atoms rather than at distant oxygen radical sites. Vanadium-rich or oxygen-poor compositions prefer symmetric adamantane-like cage structures, some of which have already been proposed in a previous study. In contrast, vanadium-poor and oxygen-rich compositions show versatile structural motifs that cannot be intuitively derived from the symmetric cage motif. Particularly, for Ti4O10− there are several non-symmetric and distorted cages that have an up to 68 kJ mol−1 lower energy than the symmetric adamantane-like cage structure. Nevertheless, for the adamantane-like cage the simulated infra-red spectrum (within the harmonic approximation) agrees best with the experimental vibrational spectrum. The oxidative power of the (TiO2)3VO3− and (TiO2)3VO2+ clusters as measured by the energy of removing 1/2 O2 (297 and 227 kJ mol−1, respectively) is less than that of the pure vanadium oxide clusters (V2O5)VO3− and (V2O5)VO2+ (283 and 165 kJ mol−1, respectively).
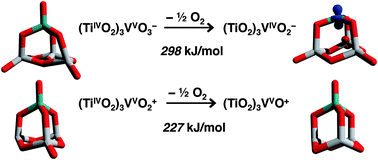

 Please wait while we load your content...
Please wait while we load your content...