Exploring zinc coordination in novel zinc battery electrolytes†
Abstract
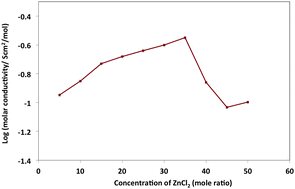
The coordination of zinc ions by tetraglyme has been investigated here to support the development of novel electrolytes for rechargeable zinc batteries. Zn2+ reduction is electrochemically reversible from tetraglyme. The spectroscopic data, molar conductivity and thermal behavior as a function of zinc composition, between mole ratios [80 : 20] and [50 : 50] [tetraglyme : zinc chloride], all suggest that strong interactions take place between chloro–zinc complexes and tetraglyme. Varying the concentration of zinc chloride produces a range of zinc–chloro species (ZnClx)2−x in solution, which hinder full interaction between the zinc ion and tetraglyme. Both the [70 : 30] and [50 : 50] mixtures are promising electrolyte candidates for reversible zinc batteries, such as the zinc–air device.

 Please wait while we load your content...
Please wait while we load your content...